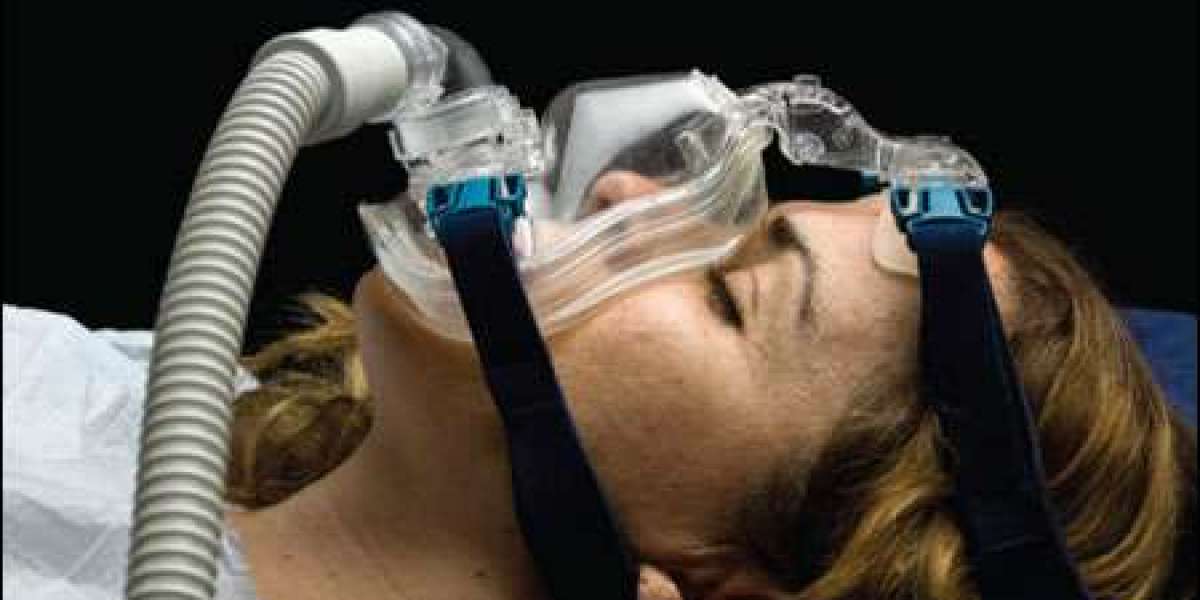
Market Overview:
According to IMARC Group's latest research publication, "Positive Airway Pressure Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2026-2034", The global positive airway pressure devices market size reachedUSD 4.0 Billionin 2025. Looking forward, IMARC Group expects the market to reachUSD 6.2 Billionby 2034, exhibiting a growth rate(CAGR) of 4.93%during 2026-2034.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
How AI is Reshaping the Future of Positive Airway Pressure Devices Market
- AI-enabled CPAP devices like ResMed's Smart Comfort received FDA clearance in December 2025, leveraging machine learning algorithms from over 100 million nights of sleep data to recommend personalized therapy settings, improving patient adherence by 25%.
- NovaResp's cMAP platform uses artificial intelligence to predict and prevent sleep apnea episodes before they occur, reducing therapy air pressure levels and enhancing patient comfort with early intervention capabilities.
- EnsoData's PAP therapy prediction AI model, EnsoTherapy, accurately predicts 30-, 60-, and 90-day patient compliance within days of starting treatment, enabling sleep coaches to provide targeted support to struggling patients.
- Wearable sensors combined with machine learning models help clinicians diagnose sleep apnea with 85% accuracy rates in home-based settings, reducing the need for traditional polysomnography and expanding diagnostic accessibility.
- AI-driven CPAP devices automatically adjust pressure based on real-time monitoring of patients' breathing patterns throughout the night, detecting changes in airway resistance and ensuring optimal pressure delivery for improved therapy outcomes.
Download a sample PDF of this report:https://www.imarcgroup.com/positive-airway-pressure-devices-market/requestsample
Key Trends in the Positive Airway Pressure Devices Market
- Surge in Telemedicine and Remote Monitoring: Connected CPAP devices with remote monitoring capabilities held over 68% share of the CPAP device market in 2024, driven by the shift toward home-based therapy. Cloud-connected modules enable healthcare providers to track patient progress, adjust therapy remotely, and offer subscription-based services for improved compliance.
- Portable and Travel-Friendly Innovations: Compact, lightweight PAP devices are gaining popularity among patients who travel frequently or desire less onerous options. React Health's Luna TravelPAP launched in March 2024 features CPAP and AutoCPAP modes, supply reminders, and optional carrying cases, representing the trend toward user-friendly portability.
- Home Healthcare Revolution: The home care segment accounted for 59.42% of the U.S. market in 2025, reflecting a major shift from hospital-based treatment. Patients favor home-based PAP therapy for convenience and cost-effectiveness, with Medicare covering 3-month trials and rental-to-own models reducing upfront costs.
- Advanced Noise Reduction and Comfort Features: Modern CPAP machines feature quieter motors below 30 dBA, compact designs, integrated humidification systems, and advanced data-tracking capabilities. These improvements address common patient concerns such as noise and discomfort, dramatically increasing therapy adherence rates.
- Digital Health Integration and Smart Technology: Automatic pressure adjustment, mobile app connectivity, and real-time monitoring are transforming PAP devices into sophisticated digital health solutions. ResMed's AirSense 11 with myAir companion app offers personalized coaching and progress tracking, making therapy more effective and user-friendly.
Growth Factors in the Positive Airway Pressure Devices Market
- Rising Prevalence of Sleep Apnea Globally: An estimated 61 million people in the U.S. suffer from obstructive sleep apnea, expected to reach 77 million by 2050. The 40.3% adult obesity rate between 2021-2023 significantly contributes to increasing OSA prevalence, driving sustained demand for PAP devices.
- Favorable Reimbursement and Insurance Policies: Government reimbursement programs including Medicare coverage for CPAP devices and accessories support market accessibility. The rental-to-own model over 10-13 months reduces upfront costs, enhances therapy initiation rates, and enables broader market penetration across patient populations.
- Growing Awareness of Sleep Disorder Risks: Public health campaigns by the CDC and healthcare organizations increased sleep apnea screenings by 11% across the United States in 2025. Growing awareness of untreated sleep apnea's links to cardiovascular disease, hypertension, diabetes, and stroke drives diagnosis and treatment adoption.
- Technological Advancements in Device Features: Auto-adjusting pressure settings, integrated humidifiers, enhanced comfort features, and built-in digital health capabilities improve patient compliance. The rental model allows payers to verify compliance before full ownership, facilitating broader acceptance and continued innovation investment.
- Expansion of Aging Population Demographics: The Congressional Budget Office projects the U.S. population eligible for Social Security will increase from 342 million in 2024 to 383 million by 2054, with significant growth in the 65+ age group who face higher susceptibility to sleep apnea disorders due to upper airway changes with aging.
Leading Companies Operating in the Global Positive Airway Pressure Devices Industry:
- 3B Medical Inc.
- Apex Medical Corp.
- Compumedics Limited (D DJ Burton Holdings Pty Ltd.)
- Drive Devilbiss Healthcare (Drive International LLC)
- Fisher Paykel Healthcare Limited
- Koninklijke Philips N.V.
- Resmed Inc.
- Smiths Group Plc
- Somnetics International Inc.
- Vyaire Medical Inc.
Positive Airway Pressure Devices Market Report Segmentation:
Breakup By Product Type:
- Automatic Positive Airway Pressure (APAP) Devices
- Continuous Positive Airway Pressure (CPAP) Devices
- Bi-level Positive Airway Pressure (BiPAP) Devices
Continuous positive airway pressure (CPAP) devices account for the majority of shares due to their proven efficacy as the gold standard treatment for obstructive sleep apnea and their cost-effectiveness compared to other PAP device types.
Breakup By End User:
- Hospitals and Sleep Labs
- Home Care
- Others
Hospitals and sleep labs dominate the market due to their role in initial diagnosis and treatment initiation, though the home care segment is experiencing the fastest growth driven by patient preference for convenient, remote treatment solutions.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position with a 46.8% market share in 2024, owing to the high prevalence of sleep apnea, advanced healthcare infrastructure, favorable reimbursement policies, and strong awareness of respiratory disorder treatment options.
Recent News and Developments in Positive Airway Pressure Devices Market
- December 2025: ResMed received FDA clearance for Smart Comfort (Personalized Therapy Comfort Settings), an AI-enabled medical device that recommends personalized comfort settings to help patients start and stay on CPAP therapy, launching in early 2026.
- July 2024: Varon introduced its Sunnygrand series CPAP devices offering effective and comfortable treatment for sleep apnea patients, integrating with doctors' prescribed settings and therapy plans while supporting ongoing monitoring and adjustments by healthcare professionals.
- May 2024: Philips launched continuous positive airway pressure (CPAP) therapy devices for sleep apnea treatment in India, offering dedicated support through a hotline and website to expand access in emerging markets.
- March 2024: React Health developed the Luna TravelPAP, a compact and lightweight travel CPAP device with CPAP and AutoCPAP modes, supply reminders, an optional carrying case, and a light ring indicator for convenient portable therapy.
- February 2024: ResMed introduced its AirCurve 11 series bilevel positive airway pressure devices in the U.S., designed to help healthcare providers treat sleep apnea while improving patient therapy initiation and adherence rates with dual pressure settings.
Note:If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email:sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302