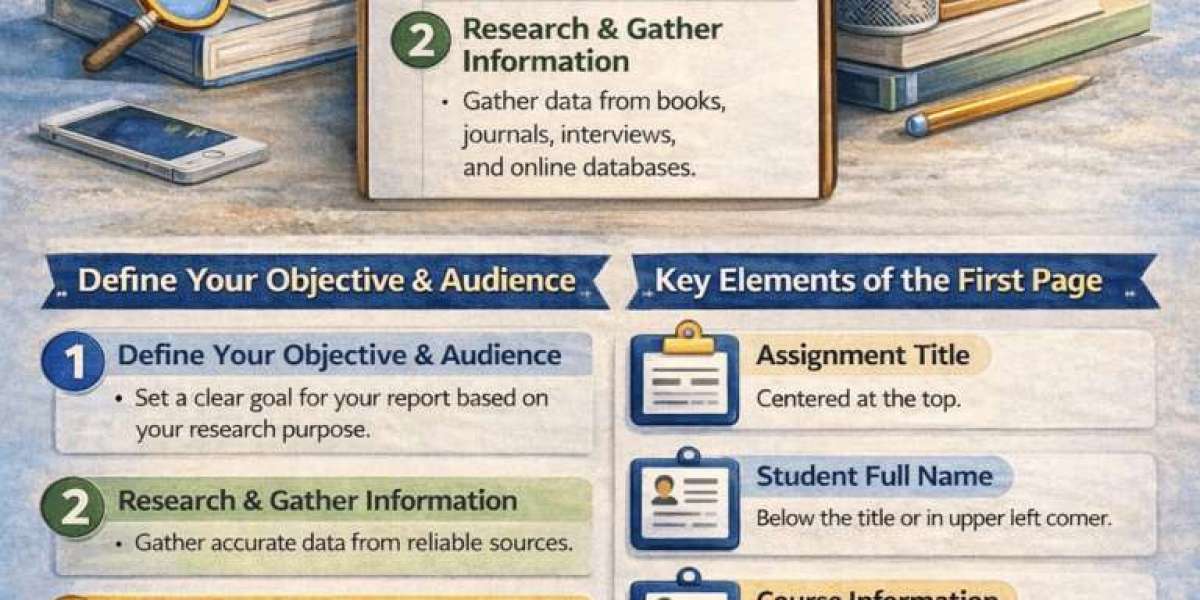
A Thermal Insulation Bottle operates on foundational principles of physics to retard the transfer of thermal energy between a liquid and its surroundings. This portable vessel achieves extended temperature retention by addressing the three primary modes of heat transfer: conduction, convection, and radiation. Its design creates a stable microenvironment, allowing hot contents to remain hot and cold contents to stay cold for many hours, serving as a practical tool for daily hydration, travel, and outdoor activities.
The effectiveness of this device hinges on its double-wall construction. A near-perfect vacuum sealed between the inner and outer walls drastically reduces heat conduction and convection, as these processes require a material medium. Often, the inner surfaces are coated with a reflective metallic layer to deflect radiant heat. The closure—a stopper or lid—completes the insulated system, forming a seal that minimizes thermal leakage at the bottle's opening. Materials like stainless steel are favored for durability, corrosion resistance, and their compatibility with maintaining a high-quality vacuum.
Selecting an appropriate Thermal Insulation Bottle involves evaluating capacity, mouth width for filling and cleaning, and lid design for drinking convenience. Advances in materials and manufacturing continue to refine performance, offering lighter weight and more durable options. Ultimately, the widespread adoption of the Thermal Insulation Bottle is a testament to its successful application of simple thermodynamic principles to solve a common, everyday challenge, providing reliable personal temperature control without external power.